Why VRAYLAR?
Is VRAYLAR right for you?
VRAYLAR works to help control the mood swings of bipolar I disorder in adults. Some medicines only treat the highs or lows. But VRAYLAR treats depressive, acute manic, and mixed episodes of bipolar I in adults. Find a way to help break through to relief. Learn more about VRAYLAR.
Have you taken any medication(s) for your bipolar I?
How does VRAYLAR work?
VRAYLAR is a once-daily capsule that provides full-spectrum relief for all bipolar I disorder symptoms: bipolar I depression, acute mixed episodes and acute mania.
While the exact way that VRAYLAR works is unknown, healthcare providers and scientists believe that VRAYLAR helps regulate dopamine and serotonin in the brain.
Watch the video to learn more about how VRAYLAR is thought to work.
Who is VRAYLAR for?
VRAYLAR is approved in adults to treat bipolar I depression and for the short-term treatment of manic or mixed episodes that happen with bipolar I disorder. While anyone can develop bipolar I disorder, it often starts in the late teen or early adult years and lasts a lifetime.

Things to keep in mind when taking VRAYLAR
Treatments like VRAYLAR start working gradually. Every patient is different, so it’s important to stick with your treatment plan. VRAYLAR is a once-daily pill that should be taken as directed by your healthcare provider. Although VRAYLAR can be taken at any time of the day, it can help to take VRAYLAR at the same time every day, which can help build a treatment routine.
Do not stop taking VRAYLAR or change how much you take without checking with your healthcare provider first.
Bipolar I stories of strength
Every bipolar I disorder journey is different. Watch real stories of real people with bipolar I disorder sharing their personal experiences.
Individual results may vary. Talk to your healthcare provider to see if VRAYLAR may be right for you.
What are some of the possible side effects of VRAYLAR?
In clinical trials, the most common side effects were difficulty moving or slow movements, tremors, uncontrolled body movements, restlessness and feeling like you need to move around, sleepiness, nausea, vomiting, and indigestion.
Patients taking VRAYLAR experienced changes in blood sugar levels and cholesterol levels similar to those who took placebo.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all possible side effects of VRAYLAR.
Please see below for additional safety information.
Will VRAYLAR cause weight gain?
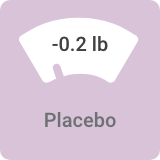
Most people taking VRAYLAR saw no substantial impact on weight¶
In VRAYLAR clinical trials, weight change reported was ≤1.5 lb.
Weight gain may occur. You and your doctor should monitor your weight regularly.
Average weight change in 6- and 8-week bipolar I depression studies:



Average weight change in 3-week bipolar I mania studies:
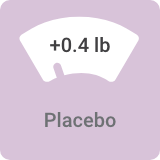


¶In clinical studies, 99% of patients in 3-week bipolar mania trials and 97% of patients in 6- and 8-week bipolar depression trials saw no substantial impact on weight (defined as ≥7% change).
Can I anticipate any sexual side effects when using VRAYLAR?
In bipolar I depression clinical studies, less than 1% of patients taking VRAYLAR reported sexual side effects.
Most common sexual adverse reactions across clinical studies#
| Placebo (n=468) |
VRAYLAR 1.5 mg/day (n=470) |
VRAYLAR 3 mg/day (n=469) |
|
|---|---|---|---|
| Abnormal orgasm | 0% | 0% | 0.4% |
| Decreased sex drive | 0% | 0.2% | 0% |
| Erectile dysfunction | 0% | 0.6% | 0.9% |
| Delayed ejaculation | 0% | 0% | 0% |
#These adverse events were self-reported by patients.
Additional resources
Tips for managing bipolar I
Discover tips that may help manage your bipolar I disorder.
*Terms and conditions apply. Available to commercially insured patients only. See Terms and Conditions for full details.